Abstract

Background
In a previous randomized trial performed by the Gruppo Italiano Trapianto di Midollo Osseo in AML patients (GITMO, AML-R2 study, Clinical Trial.gov number NCT01191957, Rambaldi A et al.: Lancet Oncology, 2015), we compared the combination of busulfan (3.2 mg/kg/die for four days) and fludarabine (160 mg/sqm) (BuFlu) with the conventional combination of busulfan and cyclophosphamide (120 mg/kg) (BuCy2). In this study, 252 AML patients with a median age of 52, undergoing allogeneic transplantation in first (n=213) or second (n=37) remission, from a sibling (n=114) or unrelated (n=138) donor were randomly assigned 1:1 to BuCy2 (n=125) or BuFlu (n=127). GvHD prophylaxis was based on cyclosporine-A and methotrexate; in case of unrelated donors, anti Thymocyte Globulin was given at a total dose of 5 mg/kg (or 7.5 mg/kg in case of HLA acceptable disparity). The 1-year non-relapse mortality (NRM) (primary endpoint of the study) of patients assigned to BuFlu proved significantly lower (7.9% in the BuFlu arm vs. 17.2% in BuCy2 arm, Gray's test p=0.026). There was no difference between the two arms in terms of cumulative incidence of relapse (CIR), leukemia free survival (LFS), overall survival (OS) at 1 and 2 years after transplant. Grade III-IV acute graft versus host disease (GvHD) was significantly higher in BuCy2 group (12 patients [10%]) than BuFlu group (3 patients [2%]). Hereby we present the long-term analysis of this clinical trial.
Methods
We collected data about the last follow up (dead/alive), the causes of death (NRM/disease relapse), the incidence of chronic GvHD (cGvHD) and current cGvHD therapy, the occurrence of disease relapse with the type of subsequent treatment including a second allogeneic transplant. We also searched for long-term side effects including cardiovascular events, secondary malignancies, other relevant medical events (i.e. infections, neurological or pulmonary events etc.). The Endpoints of the current analysis were NRM, CIR, LFS, OS, cumulative incidence of cGvHD, cumulative incidence of moderate/severe cGvHD, cGvHD and leukemia-free survival (GLFS).
Results
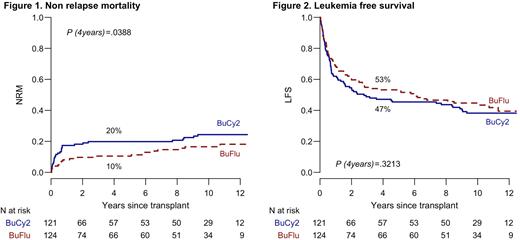
With a median follow up time for the whole study population of 6 years (range 0.03-13), the 4-years NRM is 10% in BuFlu arm vs 20% in BuCy2 arm (P= 0.0388). The 4 and 10-years CIR was not statistically different in both arms (36% vs. 33% in in BuFlu and BuCy2, and 39% vs 37% in BuFlu vs. BuCy2 arm). Similarly, we did not observe any difference in terms of 4-years LFS (53% vs 47% in BuFlu and BuCy2 arm, respectively), OS (56% in both arms) and cumulative incidence of moderate/severe cGvHD (14% in BuFlu arm vs 19% in BuCy2 arm). The 4-years GLFS was 35% in BuFlu arm vs 24% in BuCy2 (P= 0.0602). The incidence of secondary malignancies was not different in the two study groups (16 events, 7 in BuCy2 arm and 9 in BuFlu arm).
Conclusion: This long-term analysis confirms the benefit in terms of NRM associated with the BuFlu conditioning which was not associated to a higher incidence of late leukemia relapse. We found a better trend in GLFS in BuFlu arm, most likely due to a lower rate of grade III/IV acute GvHD in the experimental arm. Although we did not observe any difference between the two arms, CIR was stable and high (40% in both arms at 10 years) throughout the observation period. Therefore, AML relapse remains the major concern, urging the development of new therapeutic interventions driven by post-transplant MRD monitoring and pre-emptive therapies in high-risk patients.
Disclosures
Patriarca:Janssen: Consultancy; BMS: Consultancy; Amgen: Consultancy; GSK: Consultancy; Roche: Consultancy. Pane:Incyte, BMS, Janssen, Amgen, Jazz: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Corradini:abbvie: Honoraria; amgen: Honoraria; celgene: Honoraria; gilead: Honoraria; incyte: Honoraria; janssen: Honoraria; takeda: Honoraria. Rambaldi:Celgene-BMS: Honoraria; Janssen: Honoraria; Roche: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Kite-Gilead: Honoraria; Jazz: Honoraria; ABBVIE: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Amgen: Honoraria; Omeros: Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal